Error message
Warning: file_get_contents(http://ipinfo.io/18.118.210.229/country): failed to open stream: HTTP request failed! HTTP/1.0 429 Too Many Requests in include_once() (line 656 of /home/closuretesting/public_html/sites/default/settings.php).USP 381 US Pharmacopeial Convention - General chapter <381> Elastomeric Closures for Injections
Penetrability (functionality) testing to USP 381
USP chapter 381 defines standards for the functionality testing of closures intended to be pierced by a hypodermic needle: Penetrability, Fragmentation and Self-Sealing Capacity as well as biological and physiochemical tests. Such closures are typically used as part of a vial, bottle, or pre-fill syringe package system. This chapter states test limits for Type I and Type II elastomeric closures, formulated with natural or synthetic elastomeric substances. Type I closures are used for aqueous preparations. Type II closures are typically intended for non-aqueous preparations.
The Functionality Tests cover requirements for the closure both as shipped by the closure supplier to the injectable product manufacturer (the end user), and in final ready-to-use state by the end user. It is important to document the closure being tested, including a full description of the elastomer, and any lubrication, coating, laminations, or treatments applied.
A testing machine capable of linear displacement of the crosshead in a controlled manner and capable of measuring the peak force of penetration. Chuck of similar grip to locate the hypodermic needle used to penetrate the elastomeric membrane.
The needle specified for each test is a lubricated long bevel (bevel angle 12 ± 2°) hypodermic needle (refer to ISO 7864).
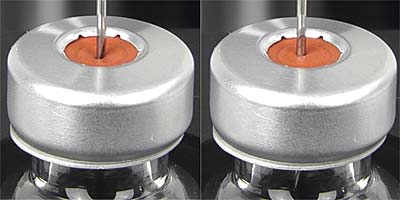
The closures for test are prepared by a process of washing and heating which also prepares a solution ("Solution S") used in other tests covered by this standard procedure. The closures are then air dried.
Fill 10 suitable vials to the nominal volume with water, fit the closures to be examined, and secure with a cap.
Using a new hypodermic needle as described above for each closure, pierce the closure with the needle perpendicular to the surface.
The force for piercing is no greater than 10 N (1 kgf) for each closure, determined with an accuracy of ± 0.25 N (25 gf)
This overview is intended to provide a basic understanding of the test procedure and suitable equipment to meet the standard. Please refer to the latest official USP Pharmacopeial Convertion website for more detailed information.
Contact Mecmesin

Got a question about this product?
Our technical sales engineers can help find the right solution for your testing requirements and provide online equipment demos with your samples.